Molnupiravir Corona, Zjojbcrbbepdtm
Molnupiravir an Oral Antiviral Treatment for COVID-19. Mercks molnupiravir is among the furthest along.
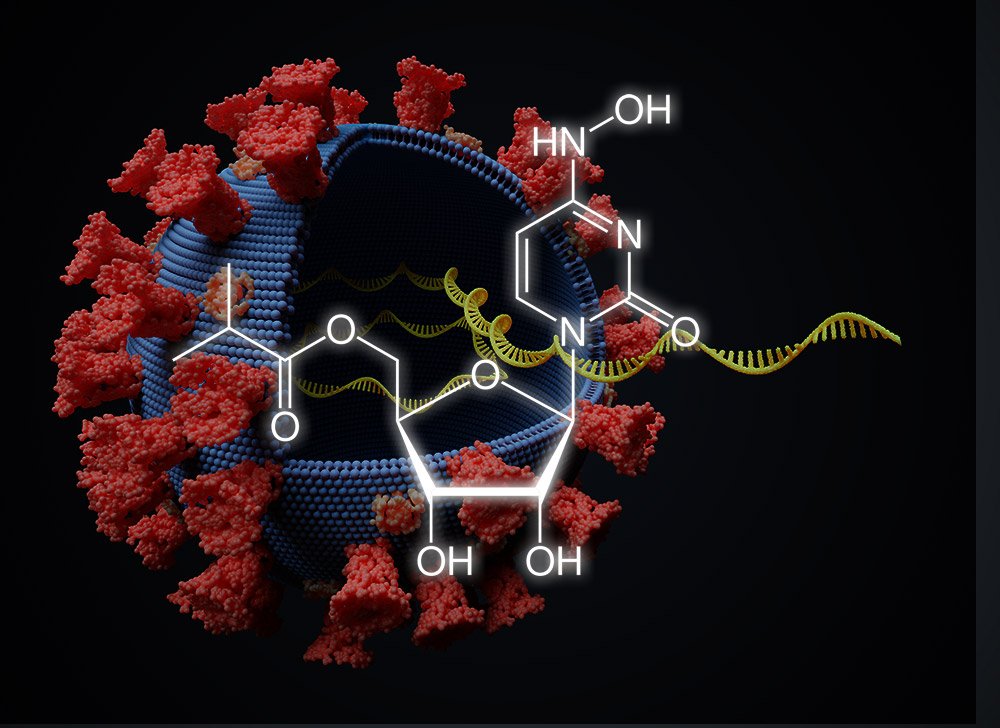
Corona Grippemittel Hemmt Coronavirus Ubertragung Antiviraler Wirkstoff Molnupiravir Blockiert Sars Cov 2 Ubertragung Bei Frettchen Scinexx De
Molecular mechanisms of corona drug candidate Molnupiravir unraveled.

Molnupiravir corona. Oral drug Molnupiravir effective against COVID-19 in hamsters. Like other antivirals molnupiravir interferes with the ability of SARS-CoV-2 the virus that causes Covid-19 to. Molnupiravir MK-4482 is designed to induce viral genome copying errors to prevent the virus from replicating in the human body and evidence to date from clinical trials in patients with COVID-19 suggests that molnupiravir may reduce replication of the SAR-CoV-2 virus.
In preliminary studies. Since the onset of the corona pandemic numerous scientific projects set out to investigate measures against the new virus. Molnupiravir is currently being tested in clinical trials.
Drugmaker Merck Co Inc said on Saturday the experimental antiviral drug molnupiravir it is developing with Ridgeback Bio showed a quicker reduction in infectious virus in its phase 2a study. Neues Corona-Medikament aus den USA soll schwere Verläufe halbieren. Molnupiravir is currently being tested in clinical trials.
Following positive study results the US pharmaceutical giant Merck Co. This is the first demonstration of an orally available drug to rapidly block SARS-CoV-2 transmission. Researchers have discovered that the treatment of SARS-CoV-2 infection Covid-19 with a new antiviral drug MK-4482EIDD-2801 or Molnupiravir comple.
The antiviral agent incorporates RNA-like building blocks into the genome of the virus. MK-4482EIDD-2801 could be game-changing said. In November Merck spent 425 million to buy.
As of June 25 2021 SARS-CoV-2. The US pharmaceutical company MSD plans to apply for approval for its corona drug Molnupiravir in the US and other countries shortly. Will für sein Corona-Medikament Molnupiravir in den USA eine Notfallzulassung beantragen.
Studie lässt hoffen Neues Corona-Mittel halbiert Risiko für sehr schwere Verläufe. The drug giant licensed the anti-Covid pill molnupiravir from Ridgeback Biotherapeutics last July. 17 hours agoE t may be an important step in the fight against the corona pandemic.
Antiviral drug Molnupiravir blocks virus transmission within 24 hours claims Study. Nach Spritzen kommen jetzt womöglich auch Tabletten. Molnupiravir itself has shown strong activity against a whole list of viruses in preclinical studies - it is indeed a very interesting candidate even more so because it seems to have an unusually.
Its developers hope the pills can be prescribed widely to anyone who gets sick. Ruchika 7 Dec 2020 1100 AM GMT. Wants to apply for emergency approval for its corona drug molnupiravir in the USA.
Since the onset of the corona pandemic numerous scientific projects set out to investigate measures against the new virus. Molnupiravir EIDD-2801MK-4482 developed by Merck in partnership with Ridgeback Biotherapeutics is one of the front-runners among investigational oral antivirals for the early outpatient. April 20 2021 1416 IST PTI April 20 2021 1414 IST.
The Department of Health and Human Services announced that it would purchase from Merck 17 million doses of molnupiravir at a cost of 12 billion provided that the current trial leads to. Die Einnahme von Molnupiravir habe das Risiko im Krankenhaus behandelt werden zu müssen um die. 1 day agoDer Pharmakonzern Merck meldet positive Ergebnisse einer Studie zu einem neuen Corona-Medikament.
Study PTI April 20 2021 1414 IST Updated. The United States recently secured 17 million doses of a compound that could help to treat Covid-19 patients. The number of hospital admissions and deaths had been cut by half by taking the Continue reading Success for new.
Molnupiravir is an antiviral drug developed by Merck and Ridgeback Therapeutics. The reason for this are recent successes in the treatment of Covid 19 patients with the antiviral drug. Because molnupiravir has shown activity against strains of coronavirus that cause the common cold it may be studied for this purpose in the future or other viruses entirely.
1 day agoDer US-Pharmakonzern Merck Co. 1 day agoProblematisch an Covid-19 war lange dass es weder Impfungen noch Medikamente gab. Merck and its partner Ridgeback Biotherapeutics said on Friday that the antiviral drug halved the risk of hospitalization or fatal disease in infected patients.

Ist Molnupiravir Der Durchbruch Im Corona Kampf Forscher Erklaren Wie Das Medikament Wirkt

Antivirale Therapie Positives Und Negatives Zu Molnupiravir Bei Covid 19 Pz Pharmazeutische Zeitung
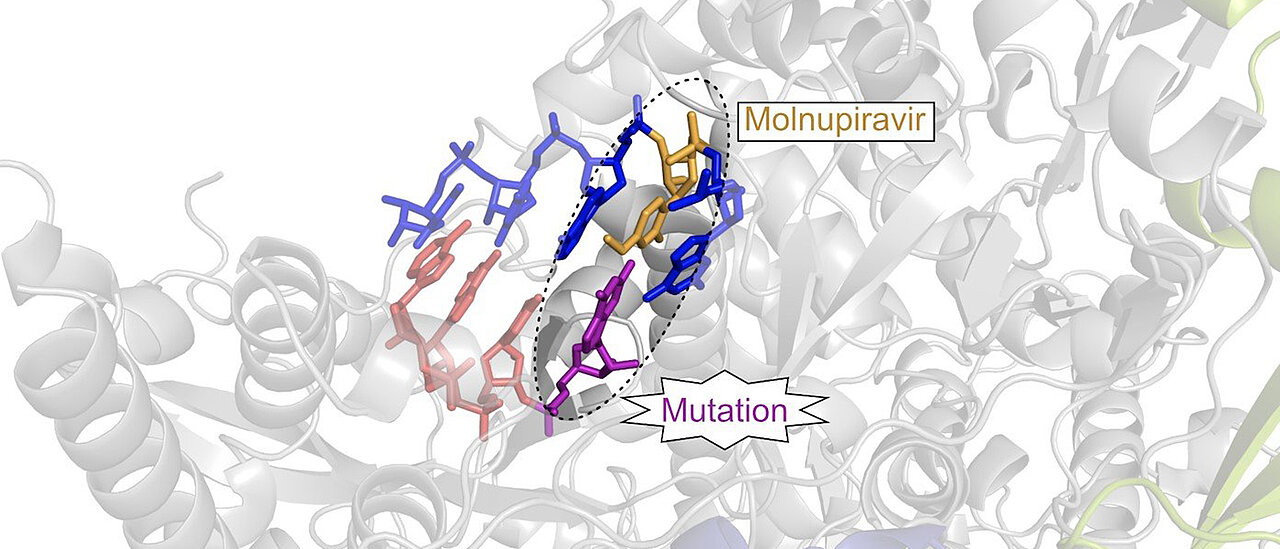
Wirkmechanismus Von Potenziellem Corona Medikament Entschlusselt Universitat Wurzburg
Corona Grippemittel Hemmt Coronavirus Ubertragung Antiviraler Wirkstoff Molnupiravir Blockiert Sars Cov 2 Ubertragung Bei Frettchen Scinexx De
Molnupiravir Neuer Corona Wirkstoff Auf Dem Weg Usa Sichern Sich 1 7 Millionen Dosen

Molnupiravir Kommt Das Wundermittel Gegen Corona Youtube

Covid 19 Hoffnungstrager Avifavir Und Favipiravir Das Sind Wir Pz Pharmazeutische Zeitung

Neue Studiendaten Molnupiravir Schutzt Mause Vor Corona Infektion Und Cov Pz Pharmazeutische Zeitung












Post a Comment
Post a Comment